Electron configuration of neon ion images are available. Electron configuration of neon ion are a topic that is being searched for and liked by netizens now. You can Find and Download the Electron configuration of neon ion files here. Download all free photos and vectors.
If you’re searching for electron configuration of neon ion pictures information connected with to the electron configuration of neon ion interest, you have pay a visit to the right blog. Our site always gives you suggestions for seeking the highest quality video and image content, please kindly hunt and find more informative video content and images that fit your interests.
Electron Configuration Of Neon Ion. With 10 electrons you should note that oxygen�s electron configuration is now exactly the same as neon�s. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. 1s2 2s2 2p6 3s2 3p6: The b atom has 2s 2 2p 1 as the electron configuration.

 79d3e15dc1b6b9f358fa12a0c5d3b97d.jpg 1,200×1,600 pixels From pinterest.com
79d3e15dc1b6b9f358fa12a0c5d3b97d.jpg 1,200×1,600 pixels From pinterest.com
Neon is a chemical element with the symbol ne and atomic number 10. 1s2 2s2 2p6 3s2 3p1: Oxygen in a neutral state would have 8 total electrons (6 valence). If you add two electrons to oxygen, it will have 10 electrons (8 valence electrons) which is exactly what neon has. Therefore the ne electron configuration will be 1s 2 2s 2 2p 6. We rewrite the electron configuration:
Because it has one unpaired electron, it is paramagnetic.
Recall, the electron configuration for na is: Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon. 1s 2 2s 2 2p 6 given : Because it has one unpaired electron, it is paramagnetic. Therefore, the abbreviated electron configuration of sodium is [ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [he]2s 2 2p 6). The electron configuration of a fluoride ion, f⁻, is _____ a.

 Source: in.pinterest.com
Source: in.pinterest.com
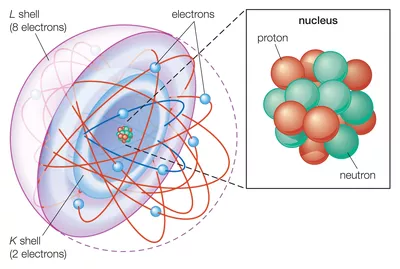
In writing the electron configuration for neon the first two electrons will go in the 1s orbital. The preceding noble gas with an atomic number less than sodium is neon, ne. It is a noble gas. Determining the valency of an element. There are 118 elements in the periodic table.
 Source: pinterest.com
Source: pinterest.com
To achieve the neon configuration, aluminum must lose three electrons, forming the al3+ ion. Neon is a chemical element with the symbol ne and atomic number 10. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation. So,this two extra electrons will be attached the. The same for na+ which has lost an electron and also is na+2,8.
 Source: pinterest.com
Source: pinterest.com
Mathematically, configurations are described by slater determinants or configuration state func Elemental fluorine has an electron configuration of 1s22s22p5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. 1s2 2s2 2p6 3s2 3p6 4s1: The electron configuration of a fluoride ion, f⁻, is _____ a. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals.
 Source: pinterest.com
Source: pinterest.com
Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. Elemental fluorine has an electron configuration of 1s22s22p5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. Electron configurations are useful for: Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital. 1s2 2s2 2p6 3s2 3p6 4s1:
 Source: pinterest.com
Source: pinterest.com
Therefore, the abbreviated electron configuration of sodium is [ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [he]2s 2 2p 6). Therefore the ne electron configuration will be 1s22s22p6. Neon is the tenth element with a total of 10 electrons. Once again, the electron configuration is the same as in the previous examples and the number of. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.
 Source: pinterest.com
Source: pinterest.com
The electron configuration is the same as for neon and the number of nonvalence electrons is 2. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. There are 118 elements in the periodic table. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
 Source: pinterest.com
Source: pinterest.com
1s2 2s2 2p6 3s2 3p6 4s2 There are 118 elements in the periodic table. Therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Neon is the tenth element with a total of 10 electrons. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.
 Source: pinterest.com
Source: pinterest.com
1s2 2s2 2p6 3s2 3p6: The remaining six electrons will go in the 2p orbital. However, there can be a few positive or negative charge ions with the same number of electrons/electron configuration as neon. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. The remaining six electrons will go in the 2p orbital.
 Source: pinterest.com
Source: pinterest.com
The br atom has 4s 2 3d 10 4p 5 as the electron configuration. 1s2 2s2 2p6 3s2 3p4: The preceding noble gas with an atomic number less than sodium is neon, ne. Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital. In its simplest form, we could write the electronic configuration of chlorine as 2,8,7 in terms of subshells, the electronic configuration would be represented as 1s 2 2s 2 2p 6 3s 2 3p 5.
 Source:
Source:
The electron configuration is the same as for neon and the number of nonvalence electrons is 2. The same as that of a neon atom Atoms and atomic structure chemistry noble gases elements and compounds science experiments. We rewrite the electron configuration: We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas.
 Source: pinterest.com
Source: pinterest.com
Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. The electron configuration is the same as for neon and the number of nonvalence electrons is 2. 1s2 2s2 2p6 3s2 3p4: Zinc�s full electron configuration is: Because it has one unpaired electron, it is paramagnetic.

This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electron configuration of neon ion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





